 Back Back
Article
ScienceAsia 32 Supplement 1 (2006): 037-042 |doi: 10.2306/scienceasia1513-1874.2006.32(s1).037
Chemical Modification of Lysine and Histidine Residues
in Gracilaria tenuistipitata Bromoperoxidase:
Effect on Stability and Activity
Jirasak Kongkiattikajorna*, Pintip Ruenwongsab and Bhinyo Panijpanb
ABSTRACT: Bromoperoxidase (BPO) from the Thai red seaweed Gracilaria tenuistipitata Chang et Xia has been
chemically modified with iodoacetamide under defined experimental conditions yielding derivatives of
native bromoperoxidase with enhanced catalytic activity. It has been shown that this modified reagent reacts
with the 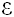 -amino groups of 15 out of a total of 46 lysine residues and 9 from a total of 16 histidine residues
of bromoperoxidase. Results obtained on kinetic parameters before and after modification are discussed in
terms of their contributions to the mechanism of irreversible thermoinactivation and activity enhancement.
The results presented in this study indicate that bromoperoxidase may acquire some new and useful
characteristics related to stability and activity upon modification of specific side chains in their primary
structures involving specific amino acids in this enzyme. -amino groups of 15 out of a total of 46 lysine residues and 9 from a total of 16 histidine residues
of bromoperoxidase. Results obtained on kinetic parameters before and after modification are discussed in
terms of their contributions to the mechanism of irreversible thermoinactivation and activity enhancement.
The results presented in this study indicate that bromoperoxidase may acquire some new and useful
characteristics related to stability and activity upon modification of specific side chains in their primary
structures involving specific amino acids in this enzyme.
Download PDF
a School of Bioresources and Technology, King Mongkut’ s University of Technology Thonburi, Thailand.
b Department of Biochemistry, Mahidol University, Thailand.
* Corresponding author, E-mail: jirasak.kon@kmutt.ac.th
| 
