 Back Back
Research Article
ScienceAsia 31 (2005): 369-381 |doi: 10.2306/scienceasia1513-1874.2005.31.369
Kinetic Modeling of Lipoprotein Peroxidation Initiated
by Copper and Azo Compounds
Somchai Yanarojana,a Udom Chantharaksri,a Prapin Wilairatb and Yongwimon Lenburyc*
ABSTRACT: Oxidation of low density lipoprotein (LDL) has been postulated as the main cause of atherogenesis,
resulting in formation of foam cells, triggering of various pathways, and leading to the development of the
disease. Therefore, antioxidants would naturally be expected to attenuate the progress of atherosclerosis. 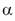 -
Tocopherol ( -
Tocopherol (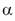 -TocH) is the most abundant form of vitamin E in nature and the major antioxidant of
biological membranes. -TocH) is the most abundant form of vitamin E in nature and the major antioxidant of
biological membranes. 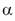 -TocH is also present at a much higher concentration than other antioxidants in
plasma lipoproteins. The large amount of -TocH is also present at a much higher concentration than other antioxidants in
plasma lipoproteins. The large amount of 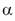 -TocH present in LDL leads to the expectation that lipid
peroxidation would be strongly inhibited by -TocH present in LDL leads to the expectation that lipid
peroxidation would be strongly inhibited by 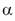 -TocH. However, advanced atherosclerotic plaques are not
deficient in the presence of antioxidants such as vitamins C and E, despite the occurrence of massive lipid
oxidation. Thus conventional mechanism associated with the antioxidant properties of -TocH. However, advanced atherosclerotic plaques are not
deficient in the presence of antioxidants such as vitamins C and E, despite the occurrence of massive lipid
oxidation. Thus conventional mechanism associated with the antioxidant properties of 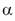 -TocH cannot
explain why lipid peroxidation occurs in atherosclerotic lesions in the presence of compounds that are usually
considered to be antioxidants. A kinetic model was developed and applied to explore the mechanism of lipid
peroxidation process under various conditions and to examine the possible occurrence of lipid peroxidation
in the presence of -TocH cannot
explain why lipid peroxidation occurs in atherosclerotic lesions in the presence of compounds that are usually
considered to be antioxidants. A kinetic model was developed and applied to explore the mechanism of lipid
peroxidation process under various conditions and to examine the possible occurrence of lipid peroxidation
in the presence of 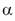 -tocopherol. The model incorporated many factors, that were not included in previous
models but we believe to play important roles in the different outcome of the process. As a result, the
numerical simulation illustrated that lipid peroxidation in the lipoprotein particle could occur in the presence
of vitamins E ( -tocopherol. The model incorporated many factors, that were not included in previous
models but we believe to play important roles in the different outcome of the process. As a result, the
numerical simulation illustrated that lipid peroxidation in the lipoprotein particle could occur in the presence
of vitamins E (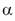 -TocH) and C under certain conditions, including high initiation rate, high initial -TocH) and C under certain conditions, including high initiation rate, high initial 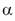 -TocH
level, low ratio of [vitamin C]/[ -TocH
level, low ratio of [vitamin C]/[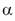 -TocH], and small lipoprotein particles. The kinetic scheme developed in
this study defined the type of relationship that -TocH], and small lipoprotein particles. The kinetic scheme developed in
this study defined the type of relationship that 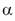 -TocH in an environment exhibits either pro- or antioxidative property. Thus, antioxidant and pro-oxidant effects of tocopherol merely depend on the condition
in which its properties are exhibited. -TocH in an environment exhibits either pro- or antioxidative property. Thus, antioxidant and pro-oxidant effects of tocopherol merely depend on the condition
in which its properties are exhibited.
Download PDF
a Department of Pharmacology, Mahidol University, Bangkok 10400, Thailand.
b Department of Chemistry, Mahidol University, Bangkok 10400, Thailand.
c Department of Mathematics, Mahidol University, Bangkok 10400, Thailand.
* Corresponding author, E-mail: scylb@mahidol.ac.th
Received 2 Mar 2005,
Accepted 21 Jun 2005
|



