 Back Back
Research Article
ScienceAsia 28 (2002) : 359-364 |doi: 10.2306/scienceasia1513-1874.2002.28.359
Spectrophotometric Determination of Astemizole and Loratadine Based on Charge-Transfer Complex Formation with Chloranilic Acid
K Basavaiah* and V S Charan
ABSTRACT: A simple and sensitive spectrophotometric method for the assay of two antihistamines, astemizole and loratadine, is described. The determination was based on the formation of chargetransfer complexes between chloranilic acid as a p - acceptor and the studied drugs as n-donors in acetonitrile solvent. The spectra, various experimental parameters, composition and formation constant of the complexes were studied. The formation constant (K) values of CT complex with astemizole were 0.9x103 and 1.06x103 calculated from Benesi-Hildebrand plot and from Turner and Anderson plot, respectively, whereas the corresponding values for loratadine were 4.50x103 and 4.19x103, respectively. The charge-transfer complexes formed were found to absorb at 520 nm. Beer’s law was obeyed in the concentration range 10 - 120 and 15 - 210 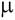 g ml-1 for astemizole and loratadine respectively. The apparent molar absorptivity and Sandell sensitivity values were 1.78x103 and 0.96x103 l mol-1 cm-1 and 257.62 and 396.61 ng cm-2, for astemizole and loratadine, respectively. The limits of detection and quantification for astemizole were 1.05 and 3.51 g ml-1 for astemizole and loratadine respectively. The apparent molar absorptivity and Sandell sensitivity values were 1.78x103 and 0.96x103 l mol-1 cm-1 and 257.62 and 396.61 ng cm-2, for astemizole and loratadine, respectively. The limits of detection and quantification for astemizole were 1.05 and 3.51 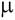 g ml-1, respectively; the corresponding values for loratadine were 2.21 and 7.39 g ml-1, respectively; the corresponding values for loratadine were 2.21 and 7.39 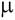 g ml-1 respectively. The method was applied to the determination of the studied drugs in several of their commercial preparations and the results were statistically validated. Excipients used as additives in commercial formulations did not interfere in the proposed procedure. g ml-1 respectively. The method was applied to the determination of the studied drugs in several of their commercial preparations and the results were statistically validated. Excipients used as additives in commercial formulations did not interfere in the proposed procedure.
Download PDF
Department of Chemistry, University of Mysore, Manasagangothri, Mysore – 570 006, Karnataka, India.
*Corresponding author, E-mail: basavaiahk@yahoo.com.in
Received 10 Oct 2001, Accepted 13 May 2002
|

