 Back Back
Research Article
ScienceAsia 27 (2001) : 239-243 |doi: 10.2306/scienceasia1513-1874.2001.27.239
Effect of Calcination Temperature on Phase Transformation and Particle size of Barium Titanate Fine Powders Synthesized by the Catecholate Process
Wongduan Maisona,*, Supon Anantab, Tawee Tunkasirib, Prasak Thavornyutikarna and Sukon Phanichphanta
ABSTRACT: A modified catecholate process has been developed for the synthesis of high purity barium titanate fine powders. The barium titanium-catechol complex, Ba[Ti(C6H4O2)3] was prepared from TiCl4, C6H4(OH)2 and BaCO3. The complex was freeze-dried and calcined for 3 hours at temperatures ranging from 700 to 1100 oC. Phase transformation and particle size of the calcined powders have been investigated as a function of calcination temperature by room-temperature X-ray diffraction and scanning electron microscopy techniques. It is seen that the perovskite-like phase of BaTiO3 was successfully obtained. With increasing calcining temperature, BaTiO3 transformed from the cubic to the tetragonal phase. Higher temperatures clearly favoured particle growth and the formation of large and hard agglomerates. After calcination at 700 oC, the average particle size ranged between 0.1-0.2 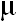 m. m.
Download PDF
a Department of Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
b Department of Physics, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
* Corresponding author, E-mail: maison202@hotmail.com
Received 13 Sep 2000, Accepted 8 Jun 2001
|

