 Back Back
Research Article
ScienceAsia 27 (2001) : 105-112 |doi: 10.2306/scienceasia1513-1874.2001.27.105
Immunoaffinity Purification and Characterization of Cyclodextrin Glycosyltransferase from Bacillus circulans A11
Jiraporn Rojtinnakorn, Pornchai Kim, Surasak Laloknam, Anchalee Tongsima, Somporn Kamolsiripichaiporn, Tipaporn Limpaseni and Piamsook Pongsawasdi*
ABSTRACT: The purified IgG fraction of anti-CGTase was coupled to CNBr-activated Sepharose 4B and used as immunoaffinity gel to purify CGTase from Bacillus circulans A11. The enzyme was successfully purified to approximately 155 folds with a 45% yield and specific activity of 3302 units/mg protein. It was a single polypeptide of 72 KDa. The amino acid composition of this CGTase was found to contain high amounts of Asx, Glx, Gly, Ala, and Thr and low amounts of His, Met, Cys, and Trp. N-Terminal sequence was A P D T S V S N K Q N F S T D V I Y Q I. Chemical modification and substrate protection studies indicate the presence of Trp, His, Tyr, and Asx/Glx residues at the active site of CGTase, while Cys, Lys, and Ser were proved to have no influence on CGTase catalysis. From HPLC analysis of products of enzymatic reaction, this CGTase produced mainly 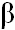 -CD. The ratio of -CD. The ratio of  : : 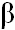 : :  - CD was 1 : 4.1 : 1.1. When analyzed by non-denaturing PAGE, the enzyme demonstrates the isoform pattern. Four isoforms with the same molecular mass but different in quantity and pI values were observed. All isoforms demonstrate amylolytic and cyclizing activities of CGTase. - CD was 1 : 4.1 : 1.1. When analyzed by non-denaturing PAGE, the enzyme demonstrates the isoform pattern. Four isoforms with the same molecular mass but different in quantity and pI values were observed. All isoforms demonstrate amylolytic and cyclizing activities of CGTase.
Download PDF
Department of Biochemistry, Faculty of Science, Chulalongkorn University, Bangkok 10330,
Thailand.
* Corresponding author, E-mail: ppiamsoo@chula.ac.th
Received 26 Jun 2000, Accepted 7 Nov 2000
|

