 Back Back
Research Article
ScienceAsia 24 (1998): 231-240 |doi: 10.2306/scienceasia1513-1874.1998.24.231
BETAINE ALDEHYDE DEHYDROGENASE FROM A HALOTOLERANT CYANOBACTERIUM APHANOTHECE HALOPHYTICA: PURIFICATION, PROPERTIES, AND REGULATION BY SALINITY
ARAN INCHAROENSAKDI AND UTAIWAN KUM-ARB
ABSTRACT: Betaine aldehyde dehydrogenase (EC 1.2.1.8) was purified from a halotolerant cyanobacterium Aphanothece halophytica. Purification was achieved by ammonium sulfate fractionation of lysozyme-disrupted cells, followed by DEAE-cellulose chromatography and hydroxyapatite chromatography. The enzyme was purified about 18-fold with a final specific activity of 298.6 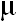 mol min-1 mg-1 protein. The enzyme was found to be a tetramer of identical 30 kDa subunits. The optima pH and temperature for the enzyme were 7.5 and 25 oC respectively. Both NAD+ and NADP+ could be used as coenzyme with Km values of 71.4 mol min-1 mg-1 protein. The enzyme was found to be a tetramer of identical 30 kDa subunits. The optima pH and temperature for the enzyme were 7.5 and 25 oC respectively. Both NAD+ and NADP+ could be used as coenzyme with Km values of 71.4 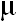 M and 100 M and 100 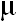 M respectively. The enzyme activity was strongly inhibited by acetaldehyde. N-methylated substrate analogs could also inhibit the enzyme activity and only slight inhibition was observed for glycine betaine. Dithiothreitol enhanced enzyme activity whereas p-chloromercuriphenylsulfonate completely abolished the activity. The enzyme was activated by KCI and NaCI at low concentrations up to 0.1 M above which the magnitude of activation was decreased for KCI and the inhibition occurred for NaCI. The elevation of external salinity resulted in the increase of the specific activity of the enzyme. M respectively. The enzyme activity was strongly inhibited by acetaldehyde. N-methylated substrate analogs could also inhibit the enzyme activity and only slight inhibition was observed for glycine betaine. Dithiothreitol enhanced enzyme activity whereas p-chloromercuriphenylsulfonate completely abolished the activity. The enzyme was activated by KCI and NaCI at low concentrations up to 0.1 M above which the magnitude of activation was decreased for KCI and the inhibition occurred for NaCI. The elevation of external salinity resulted in the increase of the specific activity of the enzyme.
Download PDF
Department of Biochemistry, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand.
Received September 25, 1998
|

